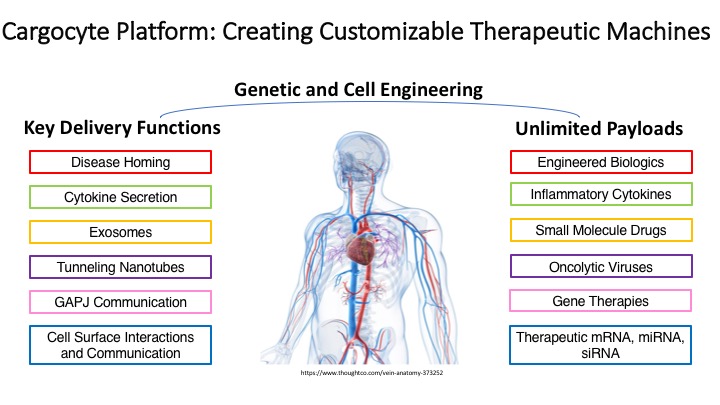
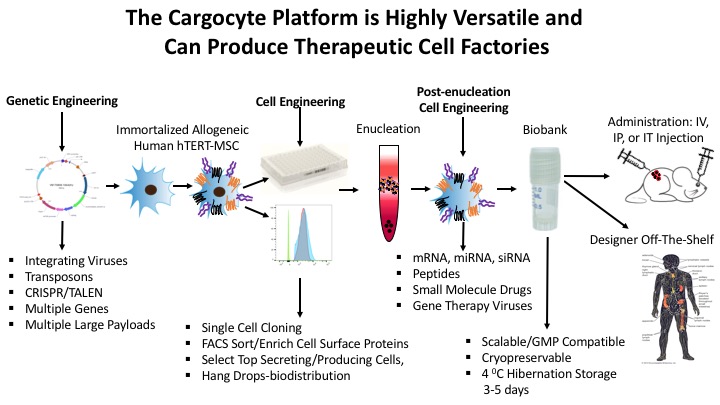
Our unique approach facilitates the rapid removal of all nuclear DNA using high speed centrifugation and Ficoll density gradients. Cargocytes™ have many benefits compared to other cell-based therapies including an excellent safety profile, a defined lifespan (3-4 days), an optimal therapeutic window (3-4 days), and can be generated from any cell type, including endogenous or exogenously-engineered autologous or allogeneic cells. Our work has also shown that Cargocytes™ are highly amenable to cutting-edge bioengineering technologies, can be engineered to precisely locate and travel to diseased tissues, and deliver various therapeutic payloads including immune modulating cytokines, RNAs, chemotherapeutic drugs, and gene therapy-encoding oncolytic viruses. Several research projects are in progress using Cargocyte™ therapeutics for the treatment of cancer and inflammatory diseases.

 |
Research Projects
Project 1. Engineering Cargocytes™ to specifically locate and home to damaged and diseased tissues and understand the underlying mechanisms that mediate this process in vitro and in vivo.
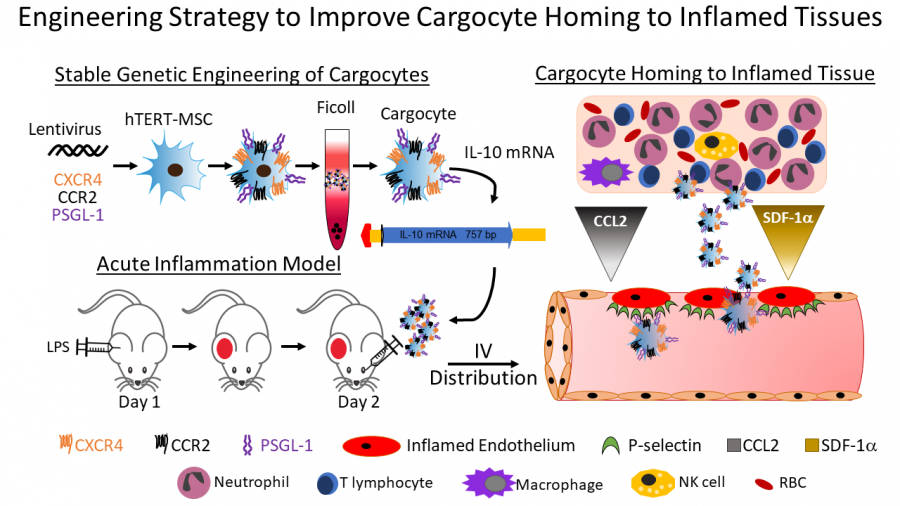
The objective of this project is to understand the underlying mechanisms that facilitate cell homing to diseased tissues and exploit these innate targeting systems to improve therapeutic cell delivery. Cell-based therapies that can home precisely to diseased tissues and can be administered intravenously is highly desired for many clinical applications. Although endogenous MSCs have robust innate ability to home to inflamed and damaged tissues, in vitro passaging and clinical-scale manufacturing of MSCs causes decreased expression of key homing receptors such as CCR2 and CXCR4. MSCs also do not express critical endothelial adhesion molecules like PSGL-1, which mediate cell adhesion to inflamed blood vessels present in many diseased tissues including malignant tumors. These deficiencies greatly impair the ability of MSCs to home to many diseased tissues, which commonly display inflamed endothelia and produce substantial amounts of CCL2 and SDF-1, the ligands for CCR2 and CXCR4, respectively. Using our unique bioengineering and enucleation protocol, the MSC-based Cargocyte™ platform provides a genetically tractable system to express specific homing receptors alone or in combination to precisely guide cells to diseased tissues following intravenous administration (Figure Right). Therefore, the major objective of this project is to stably engineer Cargocytes™ to express all three homing receptors (CCR2, CXCR4, and PSGL-1) and determine how they home to tumors and inflamed tissues using preclinical animal models. In addition, Cargocytes™ expressing these receptors will be engineered with and tested for their ability to deliver various anti-tumor and anti-inflammatory payloads in preclinical animal models of disease. Payloads under development and to be tested include antibody agonist/antagonist (CTLA-4, PD1/PD-L1, LAG3, TIM-3, CD40, etc) and immune modulatory cytokines (IL-10, TGF-β, IDO-1, etc), respectively. Also, genetic engineering of novel cytokine-cytokine and antibody targeted-cytokine fusion proteins of the above payloads are under development in the lab and will be tested using the Cargocyte™ delivery platform.
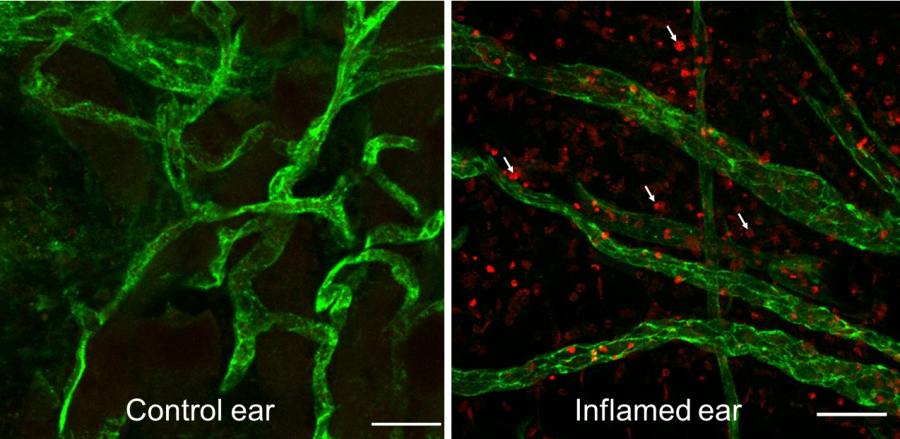 |
| Caption: hTERT-MSC-derived Cargocytes were genetically engineered to express chemokine homing receptors (CXCR4, CCR2) and the endothelial adhesion molecule PSGL-1. Cargocytes were then injected IV and allowed to home to inflamed tissue in the murine ear inflammation model as illustrated in the schematic above. Confocal images of the ear tissue reveals blood vessels (green) and numerous Cargocytes (red) that homed to the inflamed ear, exited the blood vessels, and migrated into the surrounding parenchyma. Arrows = Cargocytes. Scale bar=50 mm. |
Project 2. Developing Cargocytes™ to deliver potent immune activating cytokines to fight advanced-stage cancers and to understand the immune activating mechanisms that drive this response.
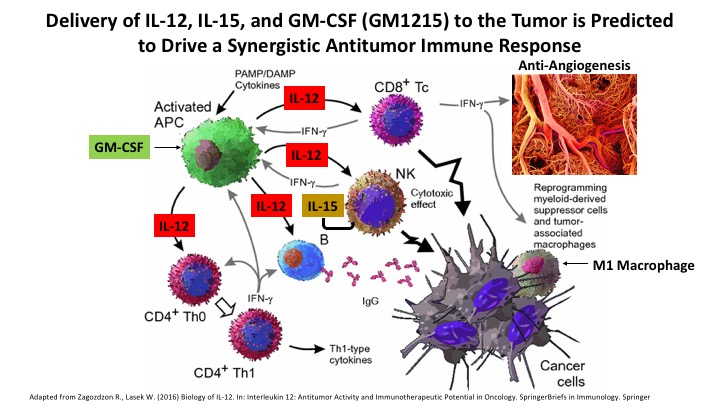
The objective of this project is to engineer hTERT-MSC-derived Cargocytes™ to simultaneously produce multiple immune activating cytokines to drive a strong local and systemic anti-tumor immune response. Using integrating lentiviruses or exogenous mRNA transfection technology Cargocytes™ are being engineered to concurrently produce therapeutic levels of IL-12, IL-15, and GM-CSF (referred to as GM1215). This specific combination of inflammatory cytokines is predicted to work synergistically to drive a strong anti-tumor immune response. IL-12 mediates enhancement of the cytotoxic activity of natural killer (NK) cells and CD8+ cytotoxic T lymphocytes, which is demonstrated by IFN-γ production and killing of target cells. IL-15 regulates the activation and proliferation of T cells and NK cells and provides survival signals to maintain memory T cells in the absence of antigen. GM-CSF plays a critical role in development and maturation of dendritic cells (DCs), proliferation and activation of T cells, and promotes DC tumor antigen presentation. Cargocytes™ producing GM1215 will be tested in immune competent preclinical mouse models of pancreatic adenocarcinoma and triple negative breast cancer. Overall, this project will determine the synergistic power of GM1215 to specifically awaken the immune system locally within the tumor and drive a systemic immune activation response directed against the primary tumor as well as metastatic lesions. Also, genetic engineering of Cargocytes™ expressing novel hybrid immune activating cytokine-cytokine and antibody-cytokine fusion proteins are under development in the lab and will be tested for anti-tumor immunity using the Cargocyte™ delivery platform.
Project 3. Engineering Cargocytes™ to deliver oncolytic viruses to advanced-stage metastatic cancers.
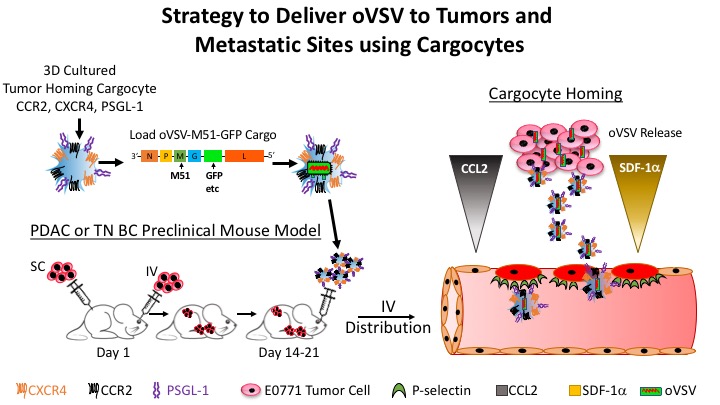
The objective of this project is to develop Cargocyte™-based therapeutics to deliver oncolytic viruses to the primary tumor and metastatic sites. In clinical trials, Oncolytic viruses have shown great promise as anti-cancer treatments for various cancers either alone or in combination with immune modulating cytokines like GM1215 (described in Project Two). Oncolytic viral therapies are rapidly evolving and can be administered either intratumorally or systemically into the vasculature. It is notable that intravascular delivery is advantageous because the virus can directly infect and target metastases, which are frequently difficult to treat in many cancers. However, current work has shown that administration of naked virus into the circulation is inefficient because the immune system rapidly clears unprotected viruses. Therefore, there is a significant need to develop biocompatible carriers that improve systemic delivery of oncolytic viruses and target them to primary and metastatic tumors. Importantly, in preclinical animal models MSC carriers display innate tumor-trophic properties, shield virus from the immune system, and can deliver oncolytic viruses to primary tumors and metastatic sites. However, the mechanisms of delivery in vivo are poorly understood and MSC homing and viral deliver need improvement for clinical applications. In this regard, hTERT-MSC Cargocytes™ expressing tumor-homing receptors (described in Project One) could substantially improve systemic administration and delivery of oncolytic viruses by shielding them from immune recognition until reaching the target tissue. Therefore, the major objective of this project is to engineer Cargocytes™ with specific tumor targeting receptor combinations (e.g. CCR2, CXCR4, and PSGL-1, etc) and determine their abilities to transport oncolytic viruses through the circulation to tumors and metastatic nodules in the body using preclinical animal models of cancer. Oncolytic viruses currently being tested include herpes simplex virus, measles virus, and vesicular stomatitis virus. These viruses have also been or will be engineered to produce various immunomodulatory agents such as IL-12, GM-CSF, and IFN-β, which boost anti-tumor immunity. Finally, Cargocytes™ armed with these oncolytic viruses are being tested for anti-tumor immunity alone or with GM1215 in combination with CTLA-4 and/or PD1/PD-L1 blockade.
|
RFP-E0771 cells (red) were infected with the oncolytic virus oVSV-M51Δ-GFP (green) at MOI 0.01 for 1h at 37 C then stained with the nuclear dye Dapi (blue). Time-lapse images were taken for 20 hr at the interval of 20 min under Nikon Eclipse-Ti microscope. Images were saved as AVI video at frame rate 12 fps using Fiji ImageJ. The appearance of green signal indicates virus infection and propagation within E0771 tumor cells which are observed to round up, bleb, and die. |